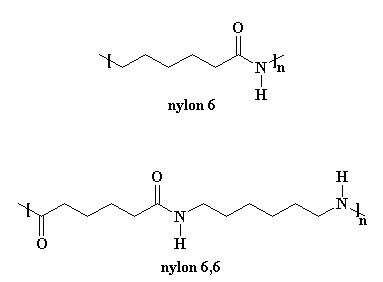
Polymer Name of Monomers Structure of Monomers i Nylon-66 Hexamethylenediamine and adipic acid ii PHBV 3-Hydroxypentanoic acid and 3. Nylon 66 is made from two monomers adipoyl chloride and hexamethylene Diamine.

Structure Of Nylon 6 6 Nylon 6 6 Is A Polyamide A Polymer Derived Download Scientific Diagram
Draw the structure of the monomer for each of the following polymers.

. Around the same time Kohei Hoshino at Toray also succeeded in synthesizing nylon 6 It is a semicrystalline polyamideUnlike most other nylons nylon 6 is not a condensation polymer but instead is. Concept Notes Videos 725. Although Nylon 6 and Nylon 66 are the most commonly seen polyamides all of the various Nylons including 8 11 12 6-9 and 6-10 have been studied extensively.
Kavungya answered the question on May 23 2019 at 1340. The chemical formula of nylon is C6H11NOn. Meltzer Mixing tank Filter Autoclave Condensation polymerization Melt spinning Drawing Texturization.
Nylon 6 is produced by ring-opening chain-growth polymerization. What is the repeating unit How many monomers are in the strand Nylon 66 is made by combining two different molecules a diacid and a diamine. A Write the structure of Nylon 66 and circle the amide linkage B Give the names and structures of the two monomers involved.
The monomeric repeating unit of nylon -6 is. Nylon 6 6 is a condensation polymer whose structure is as follows. Textbook solution for ORGCHEM EBOOK WBBWILEY PLUSCUSTOM 2nd Edition Klein Chapter 274 Problem 16ATS.
5 points Structure of monomer of nylon6. I Nylon 6 ii Polypropene. Draw the structures of monomers of the following polymers.
Delhi 2012 i Nylon-6 ii Polypropene Ans. Draw the structures of the monomers in nylon 6 6. Asked Nov 8 2018 in Chemistry by Sagarmatha 545k points polymers.
CBSE CBSE Science Class 12. Nylon 6 is only made from one kind of monomer a monomer called caprolactam. Nylon 6 nylon 66 nylon 66-6 nylon 69 nylon 610 nylon 612 nylon 11.
TPEs from blends of rubber and plastics constitute an important category of TPEs. Draw the structures of the monomers in nylon 6 6. The invention provides a light oil composition that does not cause deterioration in a Nylon-based material.
This is because acid chlorides are much more reactive than acids. Making nylon 66 is even easier if you use a diamine and a diacid chloride instead of a diacid. Chemistry questions and answers.
Nylon 6 is also known as. Monomer structure of Nylon 6. Write the Names and Structures of the Monomers of the Following Polymers.
Draw the structure of a PET fragment. The diagram below represents a cell organelle. All India 2010 Bakelite Nylon-6 Ans.
Draw the structure of the monomer for each of the following polymers. A Draw the structure of the monomer that could be polymerised to give nylon-6. The reaction is done in a two-phase system.
Draw the structure of the monomer for each of the following polymers. Asked Oct 20 2018 by. In presence of water vapor and an acid catalyst at the melt.
The molecule that bonds to two other identical molecules to form a polymer is known as a monomer. Draw the structure of nylon 46. Monomer of nylon 6 is caprolactum.
Polyamide structure Nylon 6. The monomer is the repeating unit of the polymerAmong the above two compounds one is formed with the help of condensation polymerization leading to the synthetic material. Advertisement Remove all ads.
Pg1181 Amorphous nylons are transparent. C Explain briefly how the physical properties of nylon-6 are related to its structure. Write the Names and Structures of the Monomers of the Following Polymers.
Derived from one monomer which is a molecule that can be bonded to other identical molecules to form polymers. The calculated density is 115 g cm-3. Complete Step by step answer.
Nylon 46 is a commonly used industrial nylon variant that can withstand higher temperatures than nylon 66. The amine is dissolved in water and the diacid chloride in an organic solvent. B State the function of.
For nylon 6 the monomer has six carbon atoms hence the name nylon 6. Examine the structure of Nylon 66 amide bonds have been assumed to adopt E geometries. Flow Chart of Nylon-6 Manufacturing Process.
So total valence electrons in molecule will be 178 electrons. Draw the structures of the monomers in nylon 6 6. We have step-by-step solutions for.
Structure of monomer of nylon6 - 8893832 1. What is the percent yield of polymer that you obtained in the laboratory experiment. I Nylon 6 ii Polypropene.
Hence if a nylon is named nylon 6 you know that it is made from an A-B monomer and that A-B monomer has six carbon atoms. Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 66 without violating the patent on its production. Nylon 6 6 is a condensation polymer whose structure is as follows.
Each of these monomers has six carbon atoms which is. HO OH ethylene glycol O OH O HO 14. Nylon is well suited for processing via.
00 0 votes 0 votes Rate. A Name the part labelled B. B Write a balanced equation for the formation of nylon-6 from the monomer identified in part a.
32 Polyamides are made using either a single monomer with an acid group on one end and an amine on the other or two monomers one with two acidic ends and the other with two amino. And this is the case. Phenol and formaldehyde Condensation polymer.
Made from two monomers.
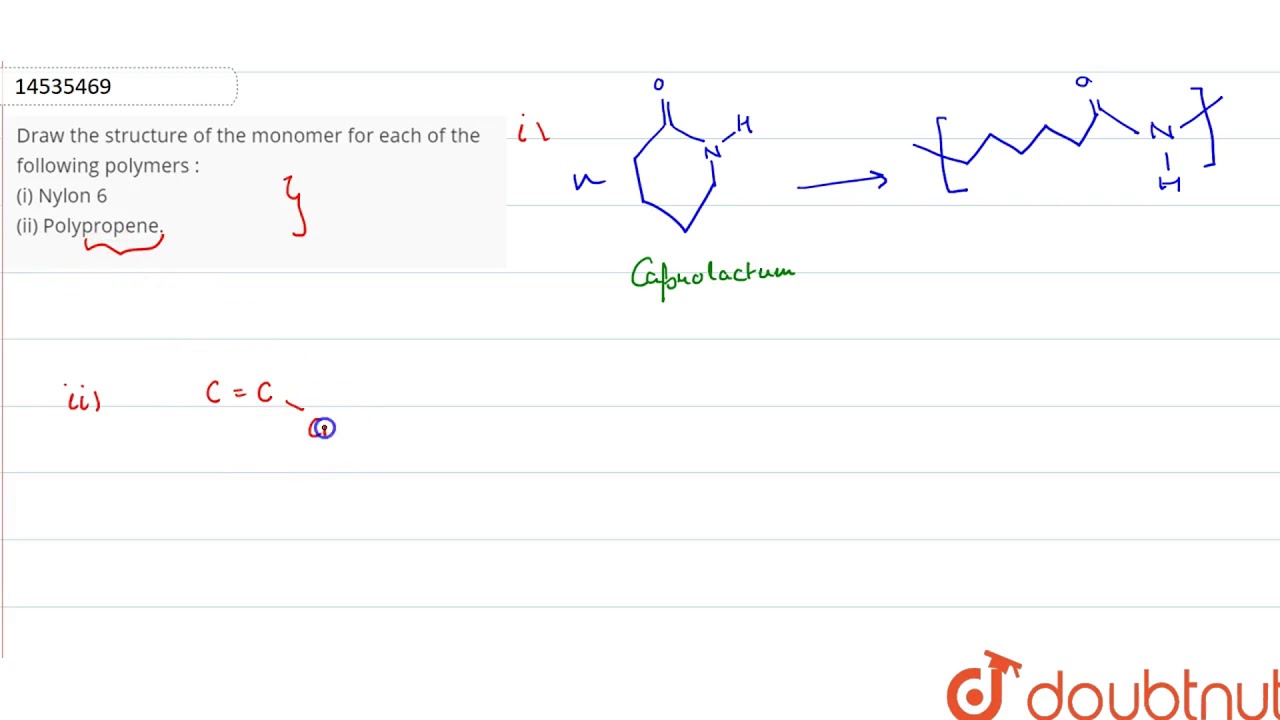
Draw The Structure Of The Monomer For Each Of The Following Polymers I Nylon 6 Ii Polypropene Youtube

Draw The Structure Of The Monomer For Each Of The Polymers I Nylon6 Ii Polypropene Youtube
Draw The Structure Of The Monomer For Each Of The Following Polymers I Nylon 6 Sarthaks Econnect Largest Online Education Community
Draw The Structure Of The Monomer For Each Of The Following Class 12 Chemistry Cbse
What Are The Monomers Of Nylon 6 And Nylon 6 6 Quora
Nylon Nylon 6 6 Plastic Polymer Chemical Structure Skeletal Formula Stock Vector Image Art Alamy
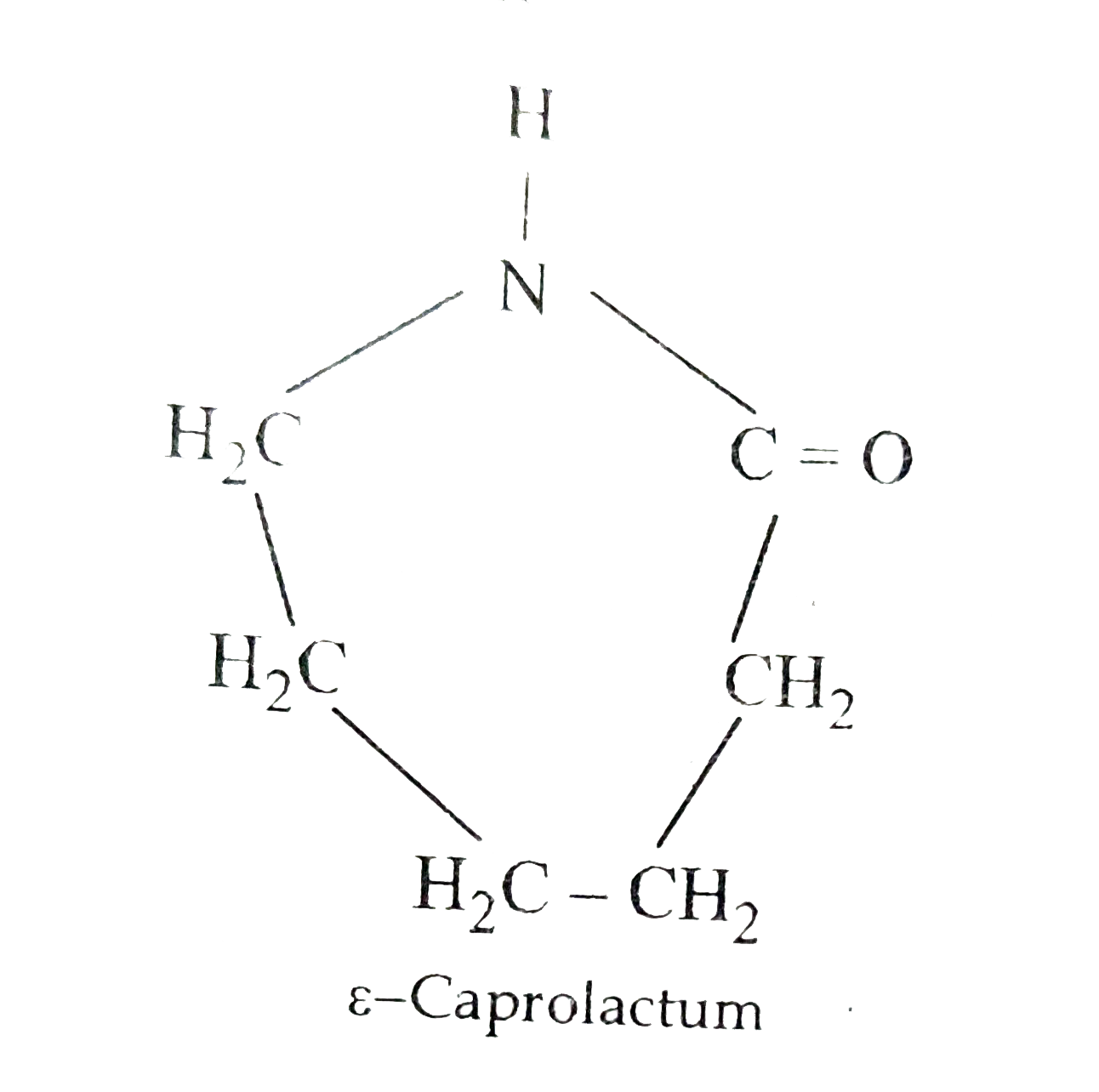
Draw The Structure Of The Monomer For Each Of The Polymers I Nylon6 Ii Polypropene
0 comments
Post a Comment